Effects of Toll-like Receptor 7 Deletion on Thymic B Cells and Antibody-Secreting Cells
Matthew Ritchie
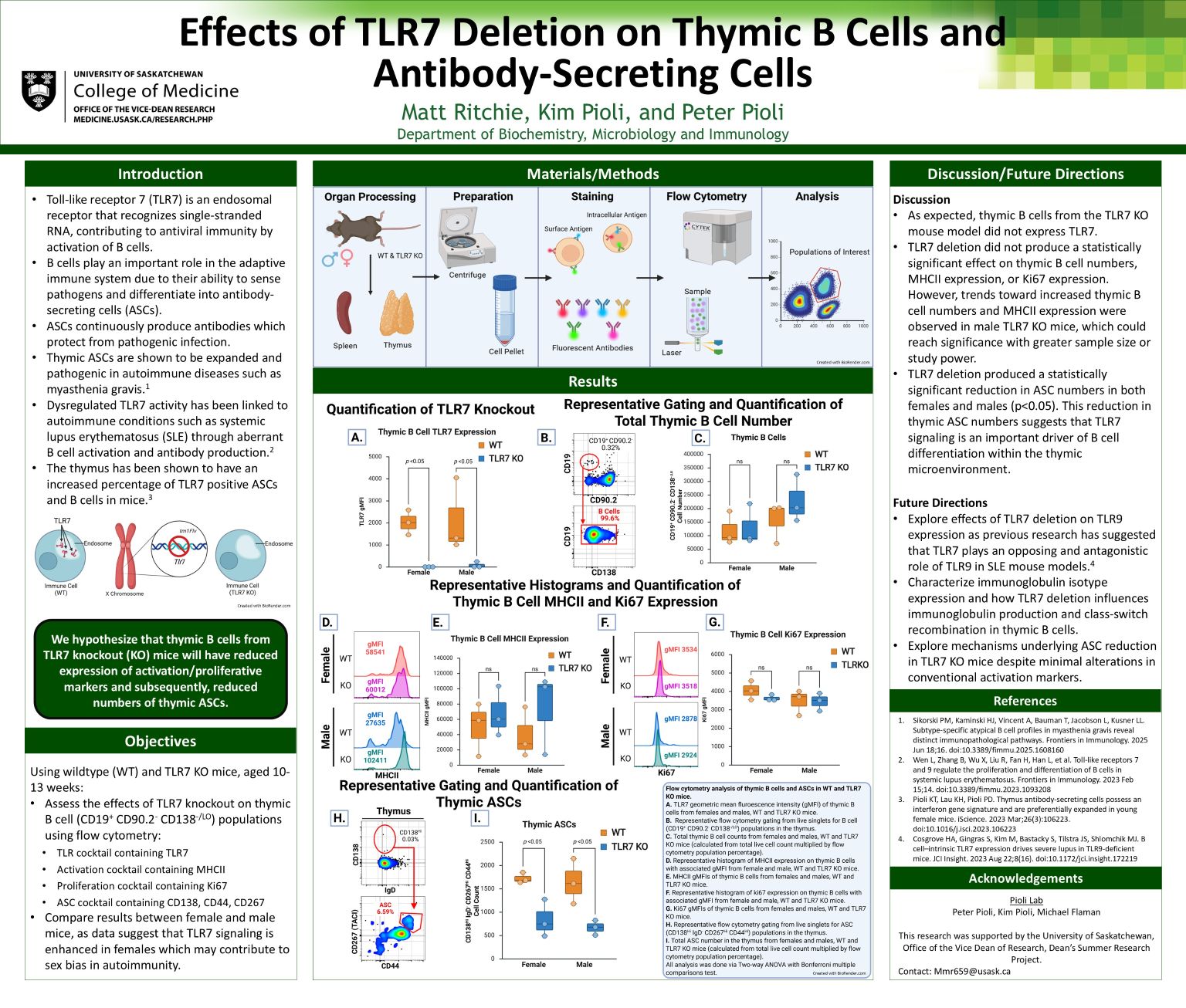
Toll-like receptor 7 (TLR7) is an endosomal RNA-sensing receptor that promotes antiviral immunity through interferon induction as well as B cell activation and differentiation into antibody-secreting cells (ASCs). While protective in infection, dysregulated TLR7 signaling is implicated in autoimmune diseases such as systemic lupus erythematosus, in part by driving aberrant ASC expansion. Thymic ASCs, which are expanded in autoimmune conditions including myasthenia gravis, may represent a pathogenic subset influenced by TLR7 signaling. We used flow cytometry to assess thymic B cell populations (CD19+CD90.2-CD138-/LO ), TLR7 expression, activation status (MHCII), proliferation (Ki67), and ASC numbers (CD138HIIgD-CD267(TACI)+CD44+) in 10–13-weeks-old wildtype and TLR7 knockout (KO) mice. Results were compared between females and males to evaluate potential sex-related differences. As expected, thymic B cells from TLR7 KO mice lacked TLR7 expression. Deletion of TLR7 did not significantly affect thymic B cell numbers or expression of MHCII and Ki67, though male TLR7 KO mice showed trends toward increased B cell numbers and MHCII expression. In contrast, TLR7 KO animals had significantly reduced thymic ASC numbers in both sexes (p<0.05). These data indicate that TLR7 is a critical driver of ASC differentiation within the thymic microenvironment.
