Deep Brain Stimulation
What is Deep Brain Stimulation?
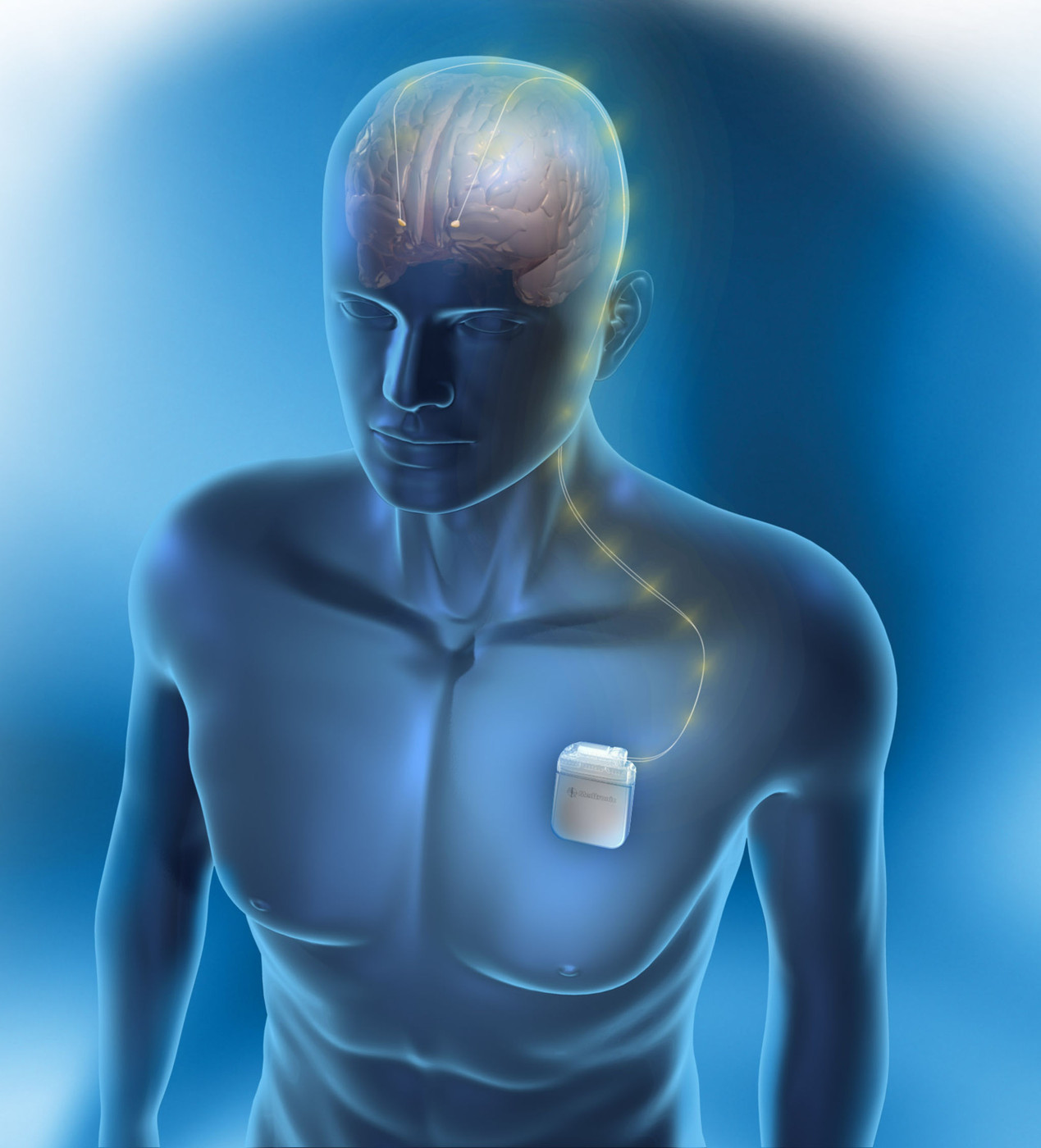
The Deep Brain Stimulation (DBS) therapy is a new treatment for a number of neurologic movement disorders as well as for chronic depression. It is sometimes called a “brain pacemaker”.
During the DBS surgery the electrodes are implanted in selected parts of the brain to deliver electrical impulses that will relieve symptoms of stiffness or uncontrolled movements.
The DBS system consists of three components: the lead (electrode), the extension cable, and the implanted pulse generator (battery).
The lead (also called an electrode) is a thin insulated wire that is inserted into the brain through a small opening in the skull (burrhole).
The extension is an insulated wire passed under the skin connecting the lead to the battery pack.
How Does it Work?
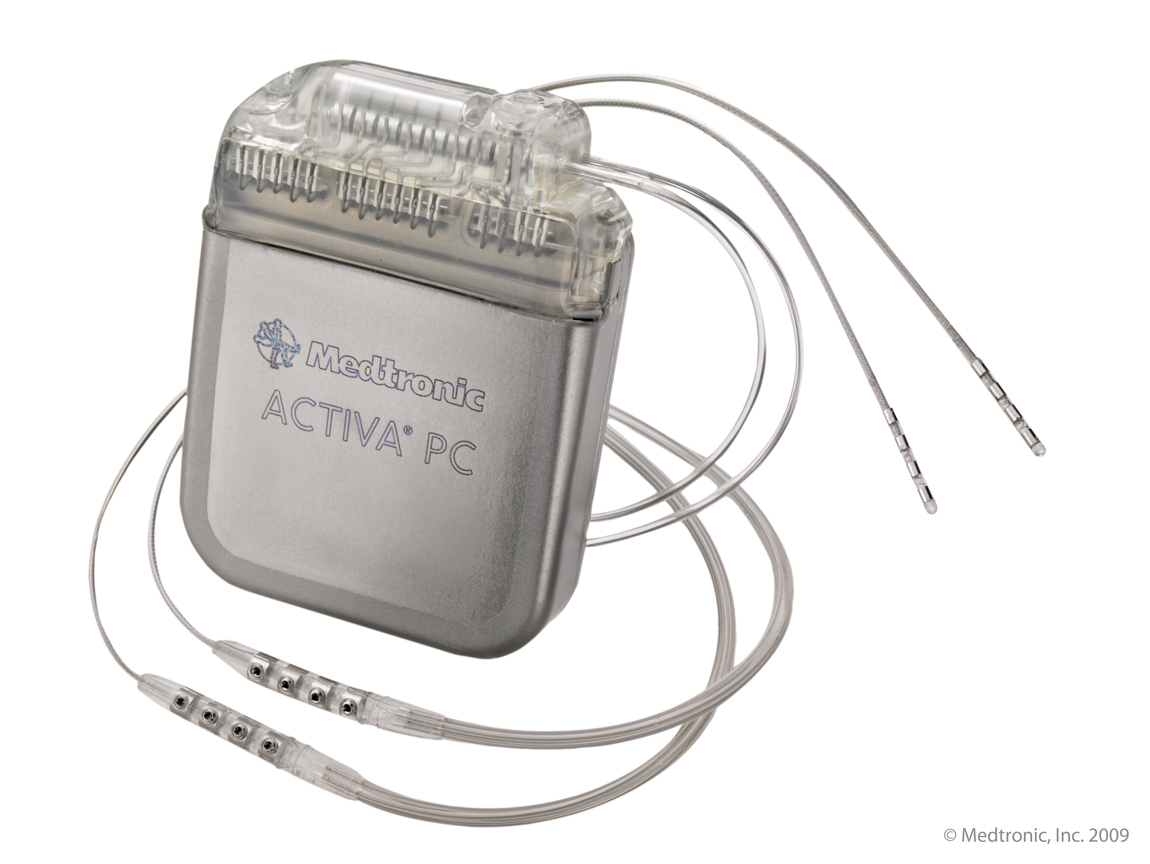
The IPG (battery) delivers constant electrical current to the tip of the electrode implanted in the brain. This stimulates a specific circuit in the brain that is abnormal in the given disease. This stimulation can significantly improve the symptoms of disease (for example tremor or stiffness). In contrast to previous operations (from which DBS have evolved), this does not damage healthy brain tissue or nerve cells. Thus, if newer more promising treatments may be available in the future, the DBS can be reversed by removal of the hardware.
Who May be a Candidate for DBS Surgery?
The main indications for DBS are otherwise treatment resistant movement disorders like Parkinson’s disease, essential tremor, and dystonia. It has been also reported to be successful in the treatment of Giles de la Tourette syndrome. Sometimes this operation may be offered for severe, chronic psychiatric conditions like depression and obsessive compulsive disorders which do not respond to medical or other forms of treatment. Treatment resistant epilepsy may be another indication.
Any patient who is not satisfied with increasing loss of control of movement arising from movement disorder may be a candidate for DBS. There are however several other factors which have to be considered before such a person may be offered a DBS treatment: the symptoms should be severe enough to cause a decline in the quality of life; the patients had an adequate and reasonable trial of medications; the medical treatment may be working but the patients exhibit severe side effects from the medications; there are no surgical contraindications to DBS treatment (like blood thinning medications, no significant brain atrophy seen on the MRI scan of the brain); the patients have the mental capability and are willing to participate in the treatment. The same would apply for patients with psychiatric disorders.
How Is DBS Surgery Done?
Before the actual surgery, the doctors need a detailed scan of the brain (MRI) to select the target in the brain where the electrode will be implanted. This is done either few days before the operation or on the morning of the surgery. For the scan the patients may need special markers (attached to the skull through small cuts in the skin), or a stereotactic frame (attached to the skull by few pins). Both are applied under local anesthetics (freezing).
Once the scan is done, the rest of the procedure is done in operating room. The patient is awake during the DBS surgery to allow the surgical team to assess the patient’s function, look for effects of the stimulation, and its side effects. Prior to the surgeon making the incision, you will be given local anesthetics (freezing). While the electrode is being advanced into the brain, the patient does not feel any pain, as the human brain does not have ability to generate pain signals.
Once electrodes are in place, the incisions on the head are temporarily closed and the frame is removed from the patient’s head. The rest of the operation is done under the general anesthetics. After the patient is asleep, the extension cable is attached to the electrode and tunneled under the skin of the head and neck to the battery which is usually placed below the collar bone.
What Are the Risks of DBS?
As with any operation, this procedure is not without the risks. The most severe (but relatively uncommon) is a brain hemorrhage. The hemorrhage may be of no significance, or may cause a stroke which will manifest as paralysis, speech impairment or even coma. The risk of a brain hemorrhage is 2-3%, the risk of stroke is less than 1%. Rarely, infections around the DBS hardware can occur. The risk is less than 5% (usually 1-2%). Treatment of infection may require removal of the whole system. Sometimes the stimulation itself can cause side effects: tingling or muscle spasms in arm or face, difficulty with speaking, tingling around the battery. Most of the time, this can be improved by a change in the stimulation settings. There is a very low risk of mechanical malfunction: the electrode or extension cable may move, break or get disconnected, the battery may fail. However this is unlikely to happen and almost always it can be fixed, but that usually means another operation. Finally the battery needs to be replaced every few years (usually after 3-5 years, but that depends on the amount of energy being used for the stimulation).
What Happens After DBS Surgery?

After the stimulator is implanted, the patient is discharged home the next day with stimulator turned off. Once the swelling from the operation settles down (which takes few weeks), the patient will be seen in the clinic to have the stimulator turned on. These settings are programmed into the implanted battery with a computer. The patients will get a patient controller which can be used to increase or decrease the stimulation, and to turn it on and off. From time to time the patients will come back to the clinic to have the stimulation fine-tuned and to check the battery life. It may take several visits before the stimulation is adjusted to one’s personal needs. About three months before the battery is expected to go dead, we will arrange for battery replacement.
If the stimulation is working well, the medications can be reduced. This will be done by Neurologist (for movement disorders) or Psychiatrist (for psychiatric conditions).
How Effective is DBS?
The effectiveness depends on the type of disorder. It can range from over 90% for limb tremor in essential tremor to about 50% for patients with epilepsy and depression. The success of the treatment also depends on the duration of the treatment: for instance for dystonia and epilepsy it can take months before the full effects of the DBS are visible.
How Can I Find Out If I Am A Candidate for DBS?
If you are suffering from one of the above listed disorders, and the symptoms are severe enough to interfere with your daily activities, or you cannot tolerate the medications, talk to your Neurologist (for movement disorders or epilepsy) or your Psychiatrist (for depression and obsessive-compulsive disorder). They can refer you to our clinic. We will meet with you and discuss the treatment in more details. We will have to assess the severity of your disease, and do some tests (like brain MRI scan), before we can tell you if you are a good candidate. Then we will discuss the operation in more detail. We will also take a video of your abnormal movement to compare it to before and after the DBS. You will also meet our Clinical Nurse Coordinator who will be able to show you some videos and answer your questions.
This treatment may be very helpful in some patients, but is not for everybody. Selecting the right patient for this therapy is a very important part of the screening process
Chronic Pain
Spinal Cord Stimulation
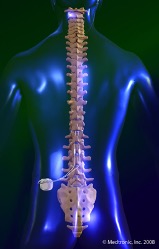
What is Spinal Cord Stimulation?
Spinal Cord Stimulation (SCS) therapy is a treatment used for some types of chronic neuropathic pain. SCS is not a cure for chronic pain. However, evidence has shown that SCS can significantly improve quality of life by reducing chronic pain and helping the individual to invest more energy into activities of daily life and rehabilitation.
During the SCS surgery a lead with a configuration of electrodes is implanted into the epidural space of your spine. This lead is connected to an implanted pulse generator (battery) by tunneling the wires under the skin.
How Does it Work?
When we have pain, the area of injury sends a signal to the brain which results in the sensation of pain. During SCS therapy the IPG (battery) sends electronic signals to the brain by stimulating the spinal cord which interrupts the pain signal. The pain signals that are blocked by the stimulator are replaced with the feeling of a gentle vibration sensation or just the absence of pain.
Potential Benefits of SCS
- Reduction in pain by greater than 50% or more
- It can help you reduce the amount of narcotics and pain medications that you use. *Any changes to medications should be done under the direction of a physician
- Increased mental clarity from decreasing medications
- Increased physical activity
- Improved overall quality of life
Who May be a Candidate for SCS Surgery?
The time to consider SCS surgery is when:
- Other treatment options have not provided adequate pain relief or intolerable or unwanted side effects
- Further surgeries are not recommended
- No surgical contraindications
What are the risks of SCS Surgery?
Spinal cord stimulation surgery is generally a safe procedure. However, as with any surgery, this procedure is not without risks. Complications of surgery may include bleeding, infection or damage to the spinal cord causing nerve injury or paralysis (rare).
What happens after SCS Surgery?
After the stimulator is implanted, the patient is seen by the nursing team for post-operative programming of the SCS battery. The patients will get a patient controller which they can use to adjust the stimulation up and down or to turn it on and off. From time to time the patients will need to come back to clinic to have the stimulation settings changed if not getting adequate pain relief.
Occipital Nerve Stimulation
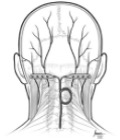
Occipital nerve stimulation involves the implantation of subcutaneous leads to stimulate the peripheral nerves in the occipital region at the base of your skull that are connected to a small battery pack in your upper chest. It is used in the treatment of Chronic Daily Headaches, Occipital Neuralgia and Intractable Chronic Cluster Headaches.
Following consultation with the Neurosurgeon, the patient will have a temporary trial of stimulation of the occipital nerve to see if this treatment is right for them. Under general anesthetic, the patient will have surgery to implant temporary subcutaneous leads over the occipital nerve attached to an external battery pack for the trial.
If the trial is determined to be successful, surgery will be planned for at least 4-6 weeks following the trial to have permanent implant of the occipital nerve stimulator. The patient will have a patient controller to adjust the stimulation settings as needed for pain relief.
Peripheral Nerve Stimulation
Peripheral nerve stimulation involves the implantation of subcutaneous leads over the peripheral nerve involved in the cause of pain connected to a small implanted battery pack. Its used in the treatment of Trigeminal Neuralgia, Chronic Facial Pain and Complex Regional Pain Syndrome.
Following consultation with the Neurosurgeon, the patient will have surgery to have temporary subcutaneous leads implanted over the peripheral nerve involved. The temporary leads are attached to an external battery pack for a trial of stimulation for 7-10 days. At the end of the trial period the temporary leads are removed in the outpatient clinic.
If the trial is determined to be successful, surgery will be planned for at least 4-6 weeks following the trial for surgery for permanent implant of subcutaneous leads and implant of battery pack for the peripheral nerve stimulator. The patient will have a patient controller to adjust the stimulation settings as needed for pain relief.
Dorsal Root Ganglion Stimulation
Dorsal Root Ganglion Stimulation (DRG) is a therapy that can be used to manage difficult to treat chronic pain in the lower part of the body such as the foot, knee, groin or pelvic area. DRG therapy works by stimulation of the dorsal root ganglia along the spinal column to try and decrease the pain to specific locations of the body.
The patient will have surgery to implant leads over the dorsal root ganglia that control the area from where the pain is located. The leads are attached using an extension cable to an external battery pack to trial the stimulation for up to 7 days to see if DRG therapy is effective for pain relief and right for you. If the trial is successful, the patient will be brought back to the OR the following week to have a permanent battery pack implanted and attached to the DRG leads. The patient will have a patient controller to adjust the stimulation settings as needed for pain relief.
Intrathecal Drug Delivery System (Pump) for Chronic Pain and/or Spasticity
What is an Intrathecal Pump?
The Intrathecal Pump is a pain or spasticity management therapy that uses a drug delivery system to deliver medication directly into the spinal fluid surrounding the spinal cord. It is typically used when oral medications no longer provide relief to pain or spasticity or intolerable side effects. The Intrathecal Pump system consists of a pump and an intrathecal catheter which are surgically implanted under the skin. Indications for use are for spasticity due to Multiple Sclerosis, Spinal Cord Injury or Cerebral Palsy.
Who may be a candidate for Intrathecal Pump Therapy?
The time to consider intrathecal pump therapy is when:
- Other treatment options have not provided adequate pain relief or intolerable or unwanted side effects
- Further surgeries are not recommended
- No surgical contraindications
- You have had a successful screening trial of intrathecal medication
About the Screening Trial?
The patient would be admitted to hospital for a trial of intrathecal medication for your pain or spasticity. A lumbar catheter will be inserted in your low back into the intrathecal space for the duration of the trial. Over the course of 2-3 days the patient will be given injections of medication by the physician through the lumbar catheter each day and the patient will be assessed for its effectiveness.
If you experience significant pain or spasticity relief during the trial and you and your doctor agree that the trial was a success you will be brought back in 4-6 weeks for implantation of the permanent intrathecal catheter and the intrathecal pump.
How does it Work?
An intrathecal drug delivery system uses a surgically implanted programmable pump in the patient’s abdomen and an implanted flexible silicone catheter that is implanted in the fluid space around your spinal cord (intrathecal space). Medication is delivered from the intrathecal pump via the catheter into spinal fluid of the intrathecal space at smaller doses than would be taken orally. The intrathecal pump will need to be refilled with the prescribed medication every 1-6 months in the outpatient clinic.
What happens after Surgery?
Following the surgery to implant the intrathecal pump and catheter your oral medications will be decreased by the physician. You will remain in hospital for 2 days postoperatively to monitor for any side effects of the intrathecal medication or any complications. Once you are discharged home, you will slowly be weaned off of your oral medications for pain or spasticity with your family physician. You may need to be seen in outpatient clinic over the next few months for adjustments of your daily dose of medication through intrathecal pump as your oral medications are decreasing. It is important to attend your appointments for your refills of your intrathecal pump as directed by the clinic.
Potential Adverse Event of Intrathecal Therapy
- Overdose or under dose of medication
- Catheter occlusion, kinking or migration
- Pump motor stall
- Pump flipping up side down
Vagal Nerve Stimulation
What is Vagal Nerve Stimulation?
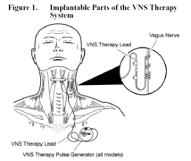
Vagal Nerve Stimulation (VNS) is a therapy approved as an add on treatment for drug resistant epilepsy and chronic depression in specific patients. It is not a cure for drug resistant epilepsy or chronic depression. VNS therapy is delivered through a battery implanted in the left chest that is connected to lead that is wrapped around the vagus nerve in the left side of the neck by delivering mild impulses throughout the day to the left vagus nerve.
Benefits of VNS Therapy
- Fewer seizures
- Less severe or shorter seizures
- Better recovery after seizures
- Improved feeling of well being
- Better mood
- Improved alertness, memory and thinking skills
- Fewer emergency room visits
About the Surgery
This surgery is only done at Royal University Hospital by Dr. A. Vitali. The surgery lasts from 1-2 hours and is done under general anesthetic. Generally patients may go home the same day. There will be a small incision on the left side of the neck and a second small incision on the left chest just below the collarbone where the battery pack is implanted.
What to Expect Following Surgery?
The patient will be seen 2 weeks after surgery in the outpatient department to have the VNS device turned on and programmed. You may need frequent visits to clinic over the first few months to have the device slowly turned up. The VNS device will cycle on and off throughout the day with the stimulation ON for 30 seconds and OFF for 5 minutes. It is important to continue to follow up with your Neurologist or Psychiatrist.
Most Common Side Effects of VNS Therapy
- Hoarseness
- Shortness of breath
- Sore throat
- Coughing
These side effects occur generally only during stimulation and decrease with time.
Learn More
Our Team
Neurosurgery
Aleksander M. Vitali, MD, FRCSC, FCS (CA) - Assistant Professor, Neurosurgery
Ivar Mendez, MD, PhD, FRCSC, FCS, DSC (HON), FCAHS - Provincial Department Head Surgery
Joesph Buwembo, MB-ChB, FRCS(SA), MMeD, FRCSC - Clinical Associate Professor, Neurosurgery
Neurophysiology
Johnathan Norton, PhD, BSC (HONS), MRES, FACNS - Assiatant Professor, Neurosurgery
Layla Gould, PhD - Assistant Professor, Neurosurgery
Karen Waterhouse RN, BSN, CNN(c)
Sara McLeod RN, BA, BScN
Cheri Derksen LPN
Tara Spencer LPN
Contacts
Dr. A. M. Vitali
Assistant Proffesor, Neurosurgery(306) 844-1102
(639)630-2107
Royal University Hospital,
103 Hospital Drive
Saskatoon SK S7N 0W8
Dr. I. Mendez
(306) 966-8201
(306) 655-2354
Provincial Departement Head, Surgery
Royal University Hospital
103 Hospital Drive
Saskatoon SK S7N 0W8
Dr. J. Buwembo
Head, Division of Neurosurgery, Regina
(306) 565-3940
(306) 966-8026
Regina SK S4P 0W5
K. Waterhouse
(306) 655-0952
(306) 655-0639
C. Derksen
(306) 966-8026
T. Spencer
(306) 966-8026
S. McLeod
(306) 655-0965
