A1.6: Dried blood spot (DBS) test for HbA1c measurement in pediatric diabetes care in Saskatchewan
Netusha Thevaranjan, Mallory McNiven, S. Flavelle, J. Robertson, J. Buse, M. Inman
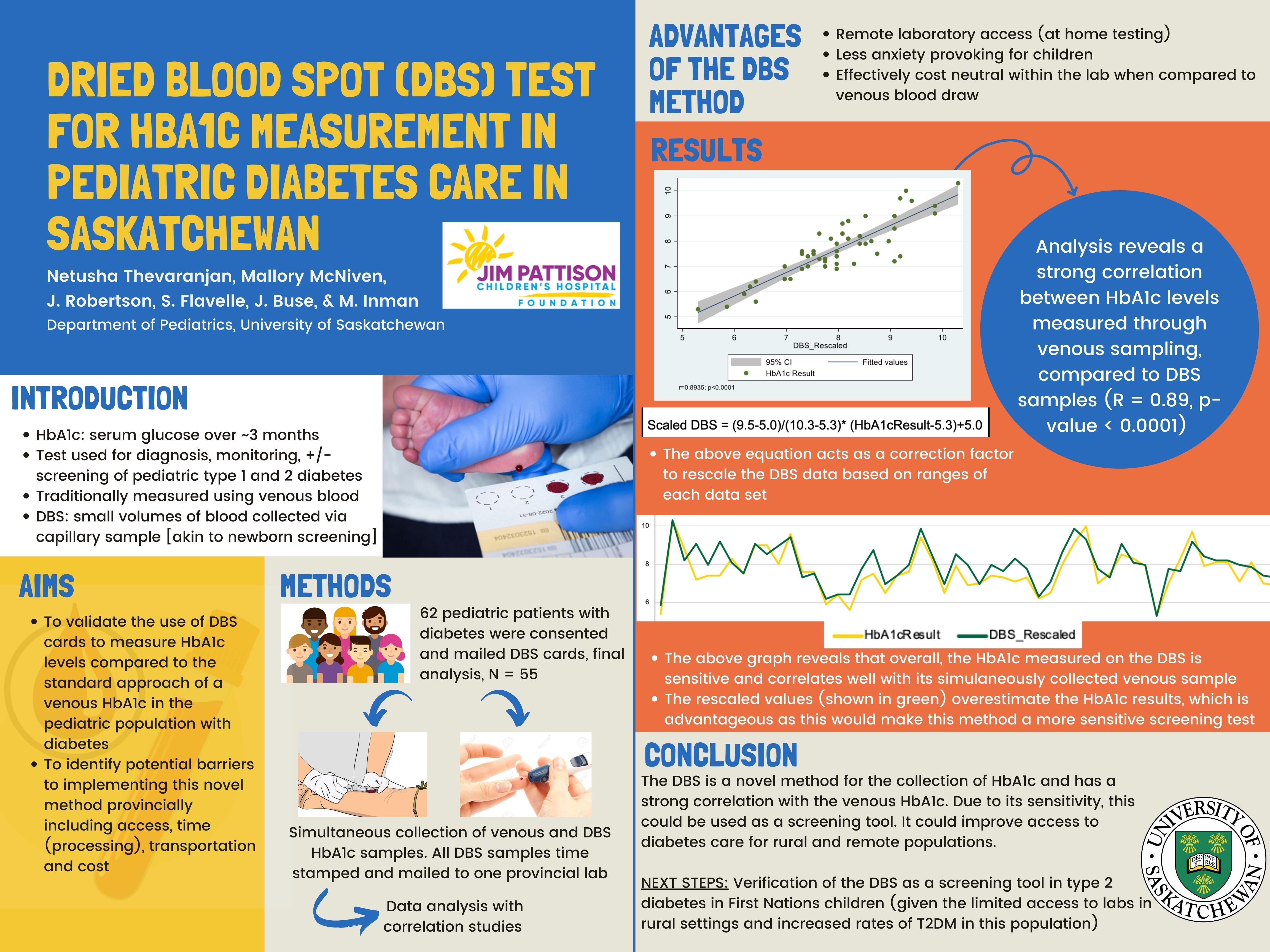
Background: Glycated hemoglobin (HbA1c) represents serum glucose over approximately 3 months and is used for diagnosis, monitoring, and screening of pediatric diabetes. However, the frequency of HbA1c testing, remote laboratory access, needle phobia, and recently COVID19 laboratory restrictions, all impede access to HbA1c testing. The dried blood spot (DBS) card is a novel method for measuring HbA1c, allowing patients to collect small volumes of blood through a capillary sample. DBS cards for HbA1c measurement have been validated in the adult population, but there is an absence of pediatric data.
Aim: This study’s aim is to validate the use of DBS cards in measurement of HbA1c in comparison to the standard venous HbA1c and to identify potential barriers to implementing this method provincially.
Methods: Venous and DBS card samples were collected simultaneously from 62 patients. Venous samples were collected as per protocol. DBS samples were collected by patients at their local laboratory, time stamped, and mailed to the provincial laboratory for single-site DBS card analysis. Correlation analyses were conducted to assess inter-assay agreement by Pearson correlation coefficient and Bland-Altman plot. The feasibility of DBS collection and processing will be assessed by time stamp analysis.
Results: 55 of 62 paired samples were analyzed (exclusions for elevated hemoglobin F, insufficient sample, and unavailable sample). Mean venous HbA1c was 7.49%; DBS was 7.26%; inter-assay difference of 0.23%. Data shows a strong positive correlation between HbA1c collection methods (r=0.87, p<0.001).
Discussion: Our study demonstrates a strong inter-assay agreement between DBS and venous HbA1c measurements. Given the DBS feasibility, cost effectiveness, and performance characteristics at a lower HbA1c, this study supports DBS testing in pediatric diabetes screening under appropriate conditions. The next phase of our research is to assess the DBS HbA1c test as a screening tool for type 2 diabetes in remote and underserved populations.
