
Dr. C. Ron Geyer MSc, PhD
Professor Department of Pathology and Laboratory Medicine- Address
- 4D01.11 Health Sciences
About
Professor, Department of Pathology and Laboratory Medicine
Associate Member, Department of Biochemistry
Ph.D., 1998, Simon Fraser University, Biochemistry
MS.C., 1992, Simon Fraser University, Biochemistry
News
Support needed for Phase II trials of life-changing Alzheimer’s treatment
In Saskatchewan, providing care for Alzheimer’s disease and related dementia costs nearly $1 billion annually. Now a new treatment could reduce that cost and provide hope to the 18,000 individuals living with the disease.
“It’s a story that’s easy to tell. It’s a good story,” Dr. Ron Geyer (PhD) says in reference to a potentially revolutionary treatment for early-stage Alzheimer’s disease. The treatment, known as NeuroEPO, showed very promising results in the first round of human trials, leading to plans for Phase II clinical trials to be conducted at the University of Saskatchewan (USask).
After a promising first round of trials, the team is preparing for the next phase by selecting trial patients and securing funding. Fundraising can be a significant barrier for clinical trials, even for something as potentially transformative as NeuroEPO.
“This team is inventing tools that we can use for diagnostics and treatment—I always tell people that I’m an inventor, not a scientist,” says Geyer. “We develop biologic therapeutics, and our specialty is antibodies and recombinant proteins. They have multiple applications, including neurodegenerative diseases.”
While there are a number of potential uses for the tools Geyer and his team are working on, treating Alzheimer’s disease is the current focus.
“This is an application that’s in dire need of a therapy. There’s no therapy on the market—there’s no cure for Alzheimer’s. The drugs that are available here have limited, short-term efficacy. They work for about three months, and then people resume cognitive decline.”
In contrast to existing drug treatments, NeuroEPO demonstrates more consistent and longer-lasting results. During the first round of human trials, 82 percent of participants receiving the new treatment experienced stabilization in their cognitive function, and more than half actually experienced an increase in their cognitive function.
Securing funding for the phase II trial would ultimately have benefits for everyone in Saskatchewan, where the provincial cost of Alzheimer’s care is currently almost $1 billion and estimated to reach $6 billion a year by 2038.
“I think it would be difficult to find somebody whose life hasn’t been touched by Alzheimer’s,” stresses Geyer.
In Saskatchewan, it’s estimated that there are approximately 18,000 people living with Alzheimer’s disease and another 20,000 people in caregiver roles. In addition to offering effective treatment to people who have been diagnosed with Alzheimer’s disease, NeuroEPO could be life-changing for their caregivers.
Geyer draws attention to another important aspect of a successful trial: improved access to diagnostic tools. Conducting the study at USask means that the research team can collaborate with other teams at the Sylvia Fedoruk Canadian Centre for Nuclear Innovation to employ best practice tools such as PET imaging and MRI measurements of brain volume.
“Using these tools can provide accurate early diagnosis and get patients onto a treatment plan before they find themselves in the hospital for other reasons,” Geyer concludes.
It’s a good story in the making, but additional support is required to make it real. To find out more about the phase II trial or contribute to this life-changing research, contact Advancement at the College of Medicine.
A new revolutionary therapy for Alzheimer’s disease
Arresting cognitive decline and improving cognition in Alzheimer’s disease
NeuroErythropoietin is a novel therapeutic drug, that has previously shown extremely promising results in human trials, arresting cognitive decline in over 80% of trial participants and improving cognition in approximately 50% of individuals with mild to moderate Alzheimer’s disease.
Dr. Ron Geyer along with neurologists, geriatricians and a clinical psychologist from the College of Medicine at the University of Saskatchewan are now advancing the development of NeuroErythropoietin to improve brain function in people with Alzheimer’s disease.
Communication in our brain occurs while neuron brain cells are in contact with other neurons. One neuron may have thousands of connections to neighbouring neurons. Signals are sent from one neuron to the neighbouring neuron to elicit chemical and electrical signals that either stimulate or inhibit activity in the neuron receiving the signal. Alzheimer’s disease typically destroys neurons and their connections in the brain region involved in memory.
Some known causes of Alzheimer’s disease involve the accumulation beta-amyloid plaques between neurons and abnormal accumulation of neurofibrillary tangles inside neurons, both of which are toxic and destructive to neurons.
Dr. Geyer’s team will conduct a phase II clinical trial in Saskatchewan, as part of Health Canada’s regulatory requirements to ultimately approve a drug, to validate the effectiveness of NeuroErythropoietin in early to mid-stage Alzheimer’s disease.
As part of the NeuroErythropoietin clinical trial, vital molecular diagnostics will be established in Saskatchewan to test for the presence and progression of beta amyloid plaques and neurofibrillary tangles, that will help clinicians more clearly diagnose Alzheimer’s disease and guide therapeutic administration.
Donate to this clinical research
Why a new Alzheimer’s disease therapy needed?
Alzheimer’s disease slowly destroys memory and thinking skills and the ability to carry out the simplest tasks. There are no effective therapies to stop the progression or to reverse the damage caused to the brain by Alzheimer’s disease.
- Nearly 1 million people in Canada live with Alzheimer’s disease.
- Nearly 190,000 new cases will be diagnosed annually in Canada by 2030.
- Canadian health care system and caregiver costs are estimated at $15 billion annually.
- In 2020 approximately 360,000 care partners were needed to care for Alzheimer’s patients in Canada.
- In Saskatchewan, 10,000 caregivers were needed in 2020 to support Alzheimer disease patients. This number will increase to 25,000 caregivers per year by 2050.
There are no effective therapies to stop the progression or to reverse the damage caused to the brain by Alzheimer’s disease. Current therapies only temporarily address disease symptoms and are ineffective as the disease progresses.
NeuroErythropoietin to treat Alzheimer’s disease
NeuroErythropoietin works by protecting neurons in the brain from death caused by toxic Alzheimer’s disease-associated molecules such as beta-amyloid while promoting the growth of new neurons and blood vessels. In clinical studies, NeuroErythropoietin improves cognitive function better than any of the currently approved Alzheimer’s disease therapies:
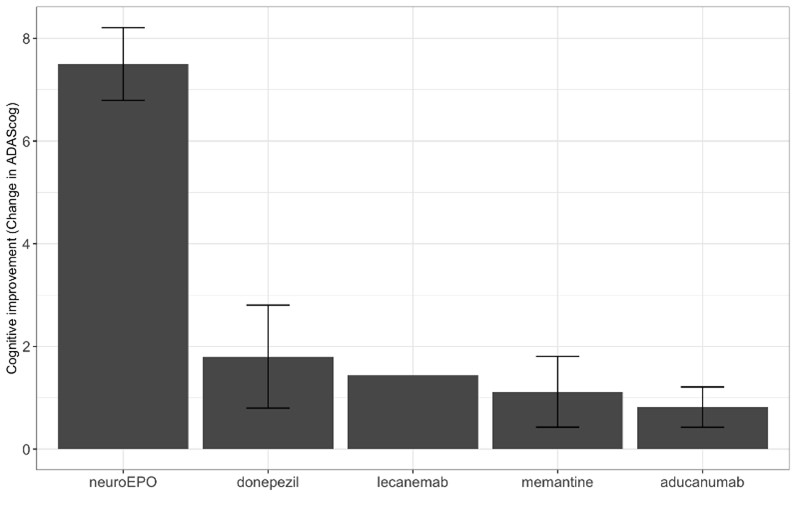
NeuroErythropoietin treatment over one year:
- Stops progression of cognitive decline in 82% of patients.
- Improves cognitive performance in 54% of patients after administration of NeuroErythropoietin.
- Increases blood flow to brain areas affected by Alzheimer’s disease in 56% of patients.
- Improves verbal fluency in patients.
Donate to this clinical research button
Contact us about this Alzheimer’s clinical research
Your donation will help us advance this new therapy for Alzheimer’s disease to the next level in the Health Canada regulatory process for approval, as well as to establish molecular diagnostics in Saskatchewan that will help clinicians to obtain diagnostic clarity for Alzheimer’s disease.
Selected Publications
- Wanpeng, S., Geyer, C.R. & Yang, J. (2008) Cloning, Expression, Purification and Crystallization of the PR Domain of Human Retinoblastoma Protein-Binding Zinc Finger Protein 1 (RIZ1). Int. J. Mol. Sci. 9, 943-950.
- Jiang, G., Freywald, T., Webster, J., Kozan, D., Geyer, C.R., DeCoteau, J.F. & Freywald, A. (2008) In Human Leukaemia Cell Lines Ephrin-B-Mediated Repulsion is Associated with Re-Assembling of Lipid Raft Signaling Complexes and Requires Lck Kinase Activity. Molecular Cancer Res. 6, 291-305.
- Lakshmikuttyamma, A., Pastural, E., Takahashi, N., Sawada, K., Sheridan, D.P., DeCoteau, J.F. & Geyer, C.R. (2008) BCR-ABL Induces Autocrine IGF-1 Signaling. Oncogene. 27, 3831-3844.
