Many faculty members within the College of Medicine are involved in research toward understanding of a variety of diseases at the molecular level. This research encompasses facets of human health and disease ranging from bacterial infections, chronic diseases to cancer and psychiatric illness. Many of these investigations require expression and characterization of proteins of interest to the researchers. The instrumentation for biophysical characterization of proteins is expensive and requires a specialized knowledge not only to operate the instruments but, most importantly, to interpret the acquired data.
In order to provide expanded support for researchers whose research requires or benefits from investigation of proteins in vitro the College of Medicine has established the Protein Characterization and Crystallization Facility (PCCF). The concept of this facility is not only to assemble the instrumentation required for protein cloning and expression, purification, characterization and crystallization but also to assure the expertise to aid the researchers in all these steps. The immediate and primary users of PCCF are the members of the PRISM Centre. However, the PCCF is open to all U of S faculty within the available instruments and personnel capacity.
The focus of the PCC Facility is on techniques specific for protein characterization and crystallization. The instruments already present include the shaker-incubators for bacterial cell cultures, the liquid handling robot (Biomek FX) for high throughput cloning and small-scale expression testing, dynamic light scattering instrument with a plate reader, crystallization robots (Gryphon and Oryx), crystallization plate hotel (CrystalFarm), isothermal titration calorimeter (nanoITC). Instruments that are on order include circular dichroism spectrometer (Chirascan Plus) and crystallization solution mixer.
PCCF is geared toward the needs of PRISM members, predominantly biologists and biochemists, and is localized in the D-wing of the Health Sciences Building in close proximity to the majority of its current and potential users. The main emphasis of PCCF is on expanding the use of biophysical methods to study proteins. While the Ph.D.-level personnel will help with setting up the experiment, their more important contribution will be to help with interpretation of the resulting data. This is a key feature of PCCF since most users are not experts in the available techniques and may have difficulty in extracting meaningful information from the obtained data.
The PRISM members will also continue accessing the instrumentation at the Saskatchewan Structural Sciences Centre (SSSC), such as the 600 MHz NMR spectrometer, mass spectrometry, and potentially the EPR instrumentation. PCCF and SSSC established already a fruitful collaboration to assure providing the best access to unique instrumentation for UofS researchers. Our joint efforts to obtain funds for instrument upgrades and replacement through CFI was very successful. The CFI grant awarded in the 2012 competition will fund among others the X-ray single-crystal diffraction instrument (located at PCCF) and Surface Plasmon Resonance instrument (located at SSSC), which will be shared by PCCF and SSSC users.
For full list of PCCF instruments visit pccf.usask.ca
Instruments
 |
Cell light pathway as low as 0.05 mm Simultaneoulsy measures CD spectra and absorbance Temperature ramping 4 °C - 100 °C up to 4 °C/min Wavelength 170nm to 1150nm Data analyzing software |
 |
With use of Dynamic Light Scattering technology proteins samples can be screened prior to crystallization trials. Experiment can provide information on purity (dispersity) of the samples, protein complexes formation, and aggregation of proteins. Temperature range 4 °C - 55 °C with temperature ramping |
 |
24K gold reaction cell of 170 µl volume |
 |
Fast set up of crystallization plates Sitting drop size as low as 0.6 µl |
 |
Automated Robot for Crystal Drop Seeding Intelliplate compatible |
 |
Crystal Farm is set-up currently to 9°C Automated imaging for crystallography condition hits (Requires VPN if access from outside UofS servers) |
 |
Dual Arm Automated Robot for Liquid Handling
96-well and 8-span arms for precise liquid dispensing
Compatible with wide range of labware
|
 |
Benchtop Liquid Handling System
Preparation of solutions for crystallography
10 ml tube format and plate format
Gradient Screen Preparation
No cross-contamination between solutions
|
 |
Surface Plasmon Resonance instrument with automated sample delivery
Two channel system
Measuring the protein-protein and protein-small molecule binding constants
Several types of chips are available from the manufacturer at a relatively low cost
|
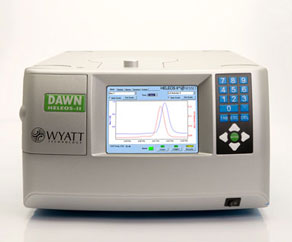 |
Multi-Angle Light Scattering System with Refracting Index Measurement Determination of molecular weight of protein in solution
Works independently or with the size exclusion column
Requires running ACTA FPLC with a programmed method
|
 |
BRUKER single crystal diffractometer with the CMOS area detector Evaluation of quality of crystal diffraction
Collecting X-ray diffraction data from protein and small molecule crystals
Crystal has to be mounted on a pin that fits the goniometer head
Temperturae of the crystal can be set between 100 K - 300 K
|
 |
Photon Technologies International Quanta Master 40 Spectrofluorometer Measure steady state rotational anisotropy (fluorescence depoloarization)
Measure steady state FRET (bulk assays)
Detects fluorophore emissions above 300 nm
For more information and to request access to instruments, contact:
|
Access
To arrange access to the PCCF instruments please contact Dr. Michal Boniecki either by email michal.boniecki@usask.ca or by phone at (306) 966-6307.
If the data obtained using the PCCF instrumentation are used for publication, please include the statement below in the acknowledgements section:
"Research described in this paper was performed in part at the Protein Characterization and Crystallization Facility, which is supported by the College of Medicine, University of Saskatchewan."
Calendar
Contact
Dr. Michal Boniecki
 |
Michal is responsible for all the instruments in PCCF. He will provide the training for the instrument you would like to use, help you in setting up your experiment and in interpreting the data. Dr. Michal Boniecki, email michal.boniecki@usask.ca, tel (306) 966-6307 |

